Anesthesia nasal mask ensures safe administration of gaseous medications
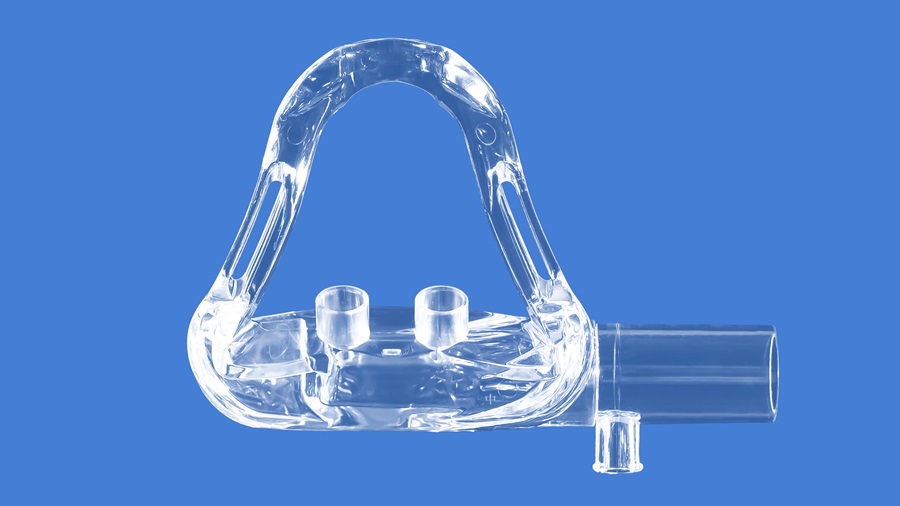
Covestro medical polycarbonate meets our high requirements for productivity and quality while complying with the latest regulatory standards ISO 18562, which will be an upcoming equivalent standard in China. Thanks Covestro China team for the onsite technical support as well as the complete material biocompatibility documents to support quick registration.
By meeting the requirements of ISO 10993, ISO 18562-3 and 18562-4, Makrolon® 2858 and was found to meet the requirements and would be well suited for use as a material in the breathing gas pathways of respiratory devices. We are honored to join hands with Nhwa to enhance the safety and quality standards of respiratory medical equipment, and together create a brighter future for patients.
Key Benefits
- High-quality Fulfills both ISO 10993 on general biocompatibility of medical device and the more recent ISO 18562 on specific biocompatibility of breathing gas pathways in healthcare applications, which is recognized by the U.S. Food and Drug Administration (FDA)
- Tough and Rigid Tough and rigid for thin wall design freedom.
- Transparent Glass-like transparency.
- Chemical-resistant Chemically resistant to gas mixtures.