Makrofol® medical grade film: clear, tough, sterile, and rigid packaging for breast implants
High-value breast implants demand high standards in packaging
The choice of pharmaceutical and medical packaging material depends on how critical the implant or device is, the value of the item itself, and the type of sterilization that the supplier or medical device manufacturer opts for. High-value breast implants have particularly stringent requirements.
A breast implant packaging solution must be able to cope with high-temperature autoclave steam sterilization that removes all residues. It must also be resistant to impacts and possess a transparency that allows for careful optical inspection of the implant prior to unpacking.
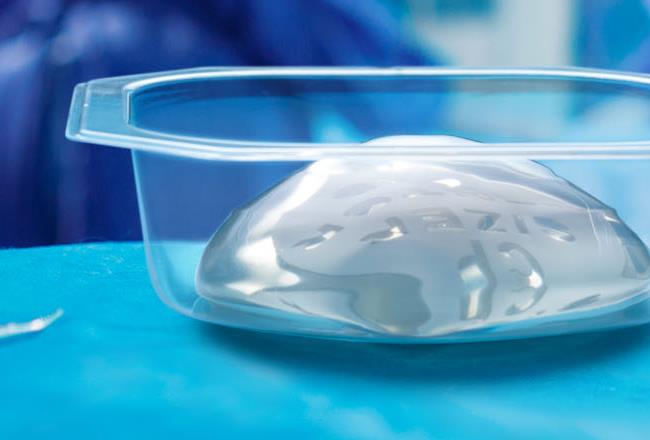
Our Makrofol® MA507 and MA336 transparent medical-grade films give surgeons a greater peace of mind. They know that the impact and crack-resistant material can withstand the most effective form of sterilization possible. And they can inspect a breast implant before they unpack it. This helps to avoid unnecessary expenses.
Makrofol® medical-grade films combine optical clarity with impact-resistant, sterilization-friendly packaging
Our high-performance Makrofol® MA507 and MA336 films are a professional choice for breast implant packaging because they provide the outstanding transparency needed for a detailed visual conformity inspection before unpacking, and are fully compatible with autoclave sterilization (temperatures of 121°C and beyond), which other polymers cannot endure. The MA336 film also allows for an additional layer of lamination.
Our medical-grade films are resistant to impact, punctures and cracking, ensuring sturdy protection for high-value healthcare items including implants. This high impact resistance coupled with the ability to withstand steam sterilization is what distinguishes transparent polycarbonate films from other medical packaging solutions.
The exceptional impact and tear resistance offered by Makrofol® MA507 and MA336 films also ensures that high-value medical items can be transported safely and arrive in the operating theater undamaged.
Both films are easy to thermoform and also comply with the ISO 9001:2015 quality management standard and two standard specifications for implantable breast prosthesis packaging of the American Society for Testing and Materials (ASTM).
Clear, rigid Makrofol® medical-grade films help surgeons to prevent implant damage and also give patients a greater peace of mind.
Key Benefits
- Sterile, residue-free: Makrofol® films withstand autoclave sterilization of 121°C and beyond.
- High safety: Sterile packaging enables an enhanced infection control and prevention for surgery.
- High optical clarity: Robust, transparent packaging enables detailed examination of the implant.
- Cost-efficient: Surgeons can inspect items before opening to avoid potential subsequent costs resulting from damaged devices.
- Impact resistant: High tear and impact resistance makes transport and handling safer.