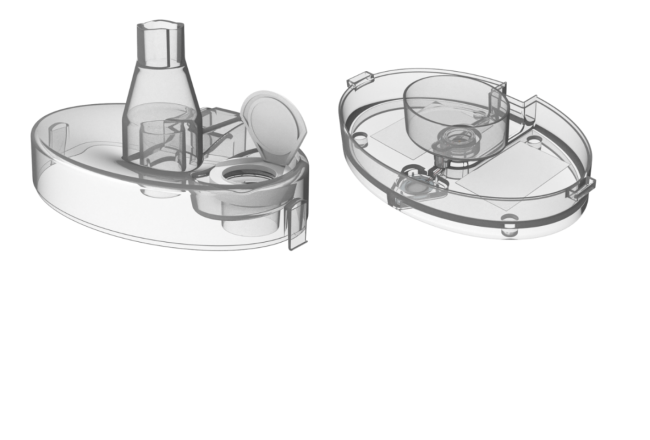
Innovative wound care device relies on Makrolon® polycarbonate
Chronic wounds afflict millions of people, and wound care is one of the most under-served markets in the medical industry. Vaporox has developed technology that is clinically validated for healing skin wounds: VHT-200. This innovative medical device received U.S. Food and Drug Administration clearance in 2023.
Vaporox needed a biocompatible, sterilizable and attractive plastic for this life-enhancing device.
“Vaporox is committed to a world where all wounds heal. Today, wound care is one of the most under-served markets in the medical industry, but this development is truly life-changing for patients and a huge step forward for this market.”
The VHT-200 features a unique combination of elements that promotes revascularization and tissue growth for those suffering from chronic skin wounds. Treatment is intended to be administered in clinics and physicians’ offices.
The company needed a robust material offering a combination of properties to form the medical device’s vaporizer component. Given the importance of this device, technical support from the plastic supplier was also an important consideration in its decision-making process.
“For decades, the healthcare market has relied on our proven portfolio of medical grade Makrolon® polycarbonate. Our work with Vaporox demonstrates the value of collaboration as our customers develop and bring truly innovative, game-changing technology to market.”
For this device, Covestro is supplying Vaporox with its Makrolon® 2458 polycarbonate, a low-viscosity, injection-molding medical grade that features easy release from the mold. Available in transparent and opaque options, this material is suitable for Ethylene Oxide (EtO) and steam sterilization and is biocompatible according to many ISO 10993-1 test requirements.
Covestro was involved throughout the device’s development, supplying prototype quantities of its material, and documentation required by the FDA.

Key Benefits
- Sterilizable Suitable for Ethylene Oxide (EtO) and steam sterilization
- Biocompatible Meets several biocompatibility criteria
- Easy to process Low-viscosity, injection-molding medical grade features easy release from the mold
- Design freedom For applications that are tough, yet attractive