QS Medical and Covestro team up again to provide a comfortable and convenient treatment experience
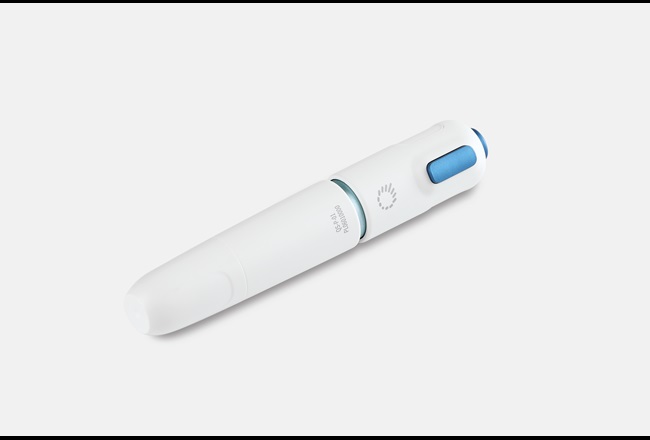
QS Medical Technology Co., Ltd. (QS), a high-tech enterprise that specializes in developing, producing, and marketing needle-free injection systems, is launching a new needle-free injection syringe that uses a high pressure fluid steam to penetrate the skin surface and deliver insulin into the subcutaneous tissue.
QS is looking for safe, high-quality, sustainable materials in the pen body and cap of this new injection syringe.
Since patients need to self-inject in different settings every day, the pen body of a needle-free syringe needs to meet certain ergonomic requirements to improve safety and convenience. The material of the pen body is also very important in order to protect the internal precision parts and to ensure effective injection.
By collaborating with Covestro again (as it did in 2017), QS launched its new needle-free injection technology, which is safe and highly effective.
In 2017, QS selected Makrolon® Rx1805, high-quality medical-grade polycarbonate of Covestro, for the ampoule, a key component in the needle-free drug delivery syringe. This year, QS again chose to cooperate with Covestro by using the latter’s PC+ABS Bayblend® in the pen body and cap to replace the ABS.
“Covestro’s Bayblend® is a preferred material for many electronic products. As a material for the body of the syringe, it has better color stability, enabling the addition of a beautiful design, and a solid, reliable structure...We look forward to continuing to work with Covestro to explore the applications of new materials in the field of healthcare products to benefit every patient and allow them better treatment experiences.”
Why Bayblend® PC + ABS is chosen for injector pen body?
- Strong and durable, high rigidity High durability and chemical resistance; high toughness even at low temperatures. High impact resistance, crack resistant.
- Color stability Compared to ABS, reduced aging from ultraviolet rays.
- Heat resistance Bayblend® FR3050 has higher heat resistance than FR-ABS, as evidenced by a significantly higher HDT. Higher HDT values help designers choose materials with more reliable dimensional stability.
- A eco-friendly product Using eco-friendly non-halogenated raw material to fulfill UL94 V0@1.5mm, which meets the flame-retardant standards of electronic medical devices and can reduce product weight using thin walls design Pre-formulated into different colors and even metallic colors , eliminating the secondary paint spraying process to achieve higher cost efficiency & quality consistency.