Case study
Makrolon® enables safer administration of dangerous drugs
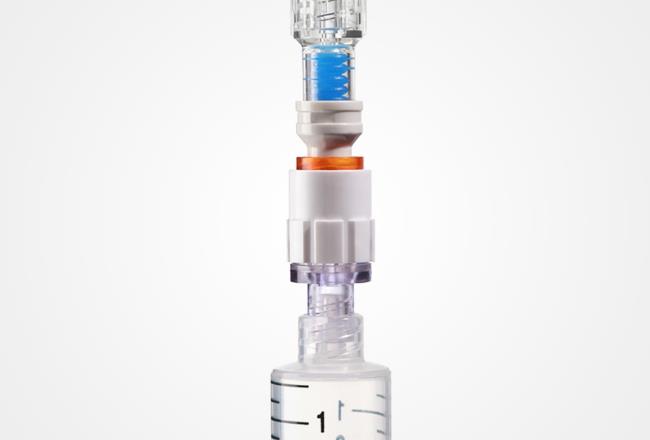
While cytotoxic drugs are vital in the treatment of some patients, inadvertent exposure can be a serious threat to the medical professionals administering them. Makrolon® Rx1805 enabled the production of a valve connector for Q-FLO™, a system that improves the safety and convenience of drug administration.
Infusion Innovations Inc. (I³) conceptualized the Q-FLO™ to improve safety and convenience for healthcare workers who need to administer cytotoxic drugs to patients. To ensure the Q-FLO™ valve connector would maintain the integrity of the sealed system, I³ needed to identify a medical grade polycarbonate material with proven safety and functionality.
I³ wanted a polycarbonate that would provide the high level of chemical resistance required for use with cytotoxic and nuclear medicines - as well as meet the regulatory standards of the U.S. Food and Drug Administration (FDA).
Makrolon®
Rx1805 offers exactly the lipid/chemical resistance I³ needed for
Q-FLO™. It also provides the clarity, strength, biocompatibility and
gamma stability needed to satisfy IS0 10993-1 quality requirements and
pass USP Class VI tests for up-to-30-day-contact with human tissue. The
FDA cleared Q-FLO™ for the administration and disposal of non-hazardous
fluids and potentially hazardous fluids such as those used in
chemotherapy, radioactive isotopes, blood products and nuclear medicine.
Why Makrolon® Rx1805 was the right solution for Q-FLO™ from I³
- Medical grade: Material resists chemicals/lipids and has high strength.
- Biocompatible: Meets ISO 10993-1 test requirements for contact with the human body.
- Radiation friendly: Suitable for sterilization with high-energy radiation.
- Transparent: Glass-like transparency makes use of the medical device easier.