Case study
Biocompatible Makrolon® selected for bone fixation device
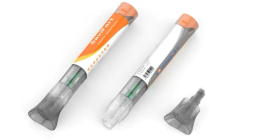
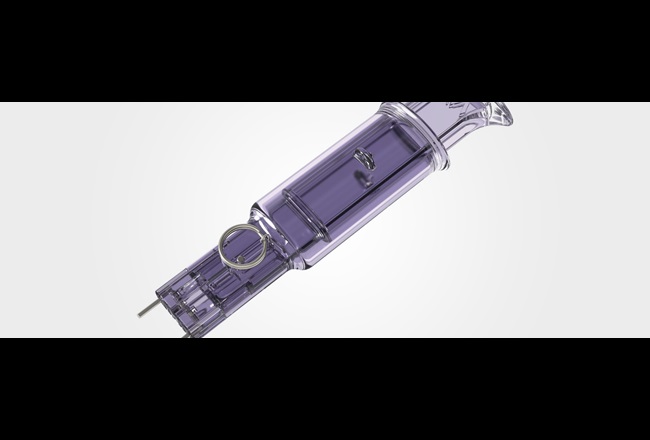
Metric Medical Devices, Inc. needed a high-strength, biocompatible polycarbonate to house their Super Staple™ Classic bone fixation device, which contributes to faster medical procedures and helps speed patient recovery. We recommended medical grade Makrolon® Rx2530.
Metric Medical Devices, Inc. was looking for a material to house their Super Staple™ Classic, a stapling device that provides compression force to improve bone healing. According to Metric, the device also reduces complications and the associated healthcare costs.
To ensure optimum convenience and cost savings for the medical profession, the Super Staple™ Classic needed to include a complete procedure kit that was strong, transparent and biocompatible, as well as sterile and disposable.
We proposed Makrolon® Rx2530 for the housing of the Super Staple™ Classic bone fixation device - because this medical-grade polycarbonate offers just the high strength, temperature stability, clarity and biocompatibility our customer needed. In addition to assisting Metric Medical Devices with material selection, we conducted tests to demonstrate the material’s compliance with ISO-10993-1 and U.S. Federal Drug Administration biocompatibility guidelines.
Why Makrolon® Rx2530 was the right solution for Metric Medical Devices
- Biocompatible: Meets the necessary ISO 10993-1 test requirements.
- Ultra-tough: Material is exceptionally durable and impact resistant.
- Dimensionally stable: Retains its original shape when exposed to heat or humidity.